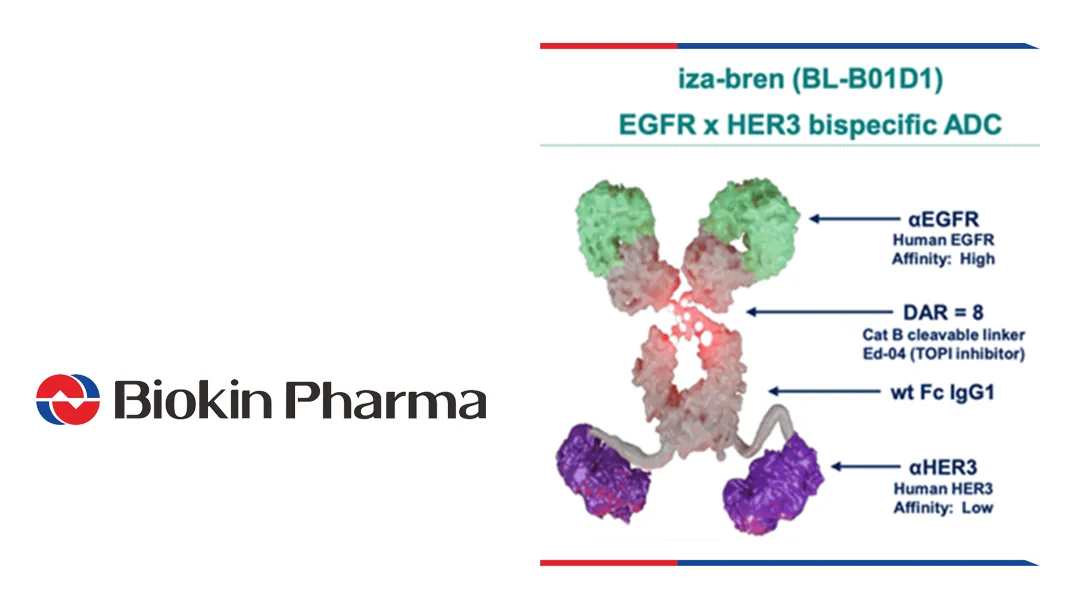
Sichuan Biokin Pharmaceutical Co., Ltd. (SHA: 688506) announced its antibody-drug conjugate iza-bren (izalontamab brengitecan, BL‑B01D1) met both primary endpoints of progression-free survival (PFS) and overall survival (OS) in a prespecified interim analysis of a Phase 3 trial for recurrent/metastatic esophageal squamous cell carcinoma (ESCC) patients who progressed after PD‑1/PD‑L1 inhibitors plus platinum chemotherapy, marking the first global Phase 3 trial where an ADC has demonstrated positive results for both endpoints in esophageal cancer.
Trial Milestone
| Item | Detail |
|---|---|
| Product | iza-bren (BL‑B01D1) |
| Mechanism | EGFR×HER3 bispecific ADC |
| Indication | Recurrent/metastatic ESCC post PD‑1/PD‑L1 + platinum chemo |
| Trial Phase | Phase 3 (prespecified interim) |
| Primary Endpoints | PFS and OS (both met) |
| Significance | Global first ADC to demonstrate dual survival benefit in esophageal cancer |
| Next Step | NDA submission to NMPA in H1 2026 |
Drug Profile
- Class: First‑in‑class, new‑concept EGFR×HER3 bispecific ADC; only candidate of its kind in Phase 3 development
- Pipeline: Under evaluation in >40 clinical trials across China and the U.S. for various tumor types
- Global Rights: Exclusive license to Bristol‑Myers Squibb (BMS) outside mainland China since December 2023; BMS funds global program
Regulatory Designations
| Agency | Breakthrough Therapy Designations |
|---|---|
| CDE (NMPA) | 7 indications (including ESCC, NSCLC, breast cancer) |
| FDA | 1 indication (breast cancer) |
Clinical Evidence Summary
- Patient Population: Recurrent/metastatic ESCC after prior checkpoint inhibitor + chemotherapy (high unmet need)
- Efficacy: Both PFS and OS primary endpoints met at interim analysis (statistical significance not yet disclosed)
- Safety: Profile consistent with prior studies; no new safety signals identified
Market Impact & Outlook
- China Market: ~320,000 new esophageal cancer cases annually; ~40% ESCC eligible for second‑line therapy
- Revenue Forecast: Analysts project peak China sales of ¥3.5‑4.0 billion (~US$480‑550 million) by 2030
- BMS Partnership: Upfront payment of US$800 million plus milestones and double‑digit royalties; BMS driving global Phase 3 program
- Competitive Edge: Only ADC with OS data in esophageal cancer; positions ahead of Merck’s MK‑2870 and Daiichi’s T‑DXd in this setting
Forward‑Looking Statements
This brief contains forward‑looking statements regarding iza‑bren’s regulatory path, commercial potential, and partnership outcomes. Actual results may differ due to risks including final trial data, NMPA/FDA review, and market adoption.-Fineline Info & Tech