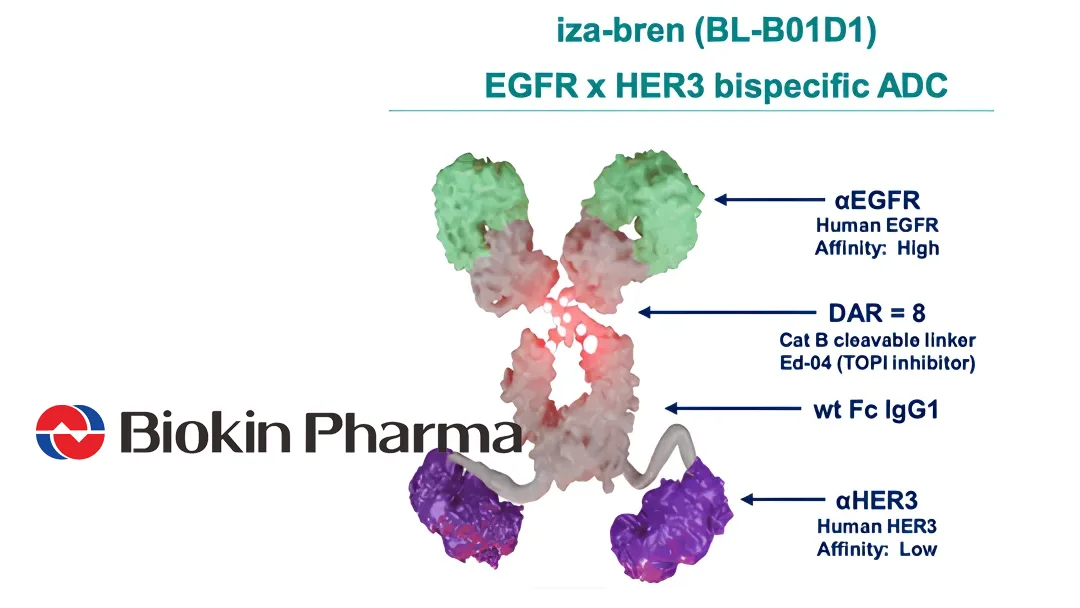
Sichuan Biokin Pharmaceutical Co., Ltd. (SHA: 688506) announced that its New Drug Application (NDA) for iza-bren (izalontamab brengitecan, BL‑B01D1), a first‑in‑class EGFR×HER3 bispecific ADC, has been formally accepted by the National Medical Products Administration (NMPA) for recurrent or metastatic nasopharyngeal carcinoma (NPC) patients who progressed after PD‑1/PD‑L1 therapy and at least two chemotherapy lines.
Regulatory Milestone
| Item | Detail |
|---|---|
| Product | iza‑bren (BL‑B01D1) |
| Company | Sichuan Biokin Pharmaceutical (688506.SH) |
| Agency | NMPA (China) |
| Application Type | New Drug Application (NDA) |
| Indication | Recurrent/metastatic NPC post PD‑1/PD‑L1 + ≥2 chemo lines (≥1 platinum) |
| Status | Formally accepted for review |
| Global Position | Only EGFR×HER3 bispecific ADC in Phase III globally |
Drug Profile & Development
- Mechanism: First‑in‑class EGFR×HER3 bispecific ADC delivering cytotoxic payload to dual‑target tumor cells
- Pipeline: >40 clinical trials ongoing in China and U.S. across multiple tumor types
- Breakthrough Designations: 7 indications on CDE BTD list (China); 1 indication on FDA BTD list (U.S.)
- Global Partnership: In December 2023, Bristol Myers Squibb acquired ex‑China rights for iza‑bren, providing $800 million upfront plus milestones
Market Impact & Outlook
| Metric | Value |
|---|---|
| China NPC Incidence | ~120,000 new cases annually |
| Recurrent/Metastatic Population | ~30% of patients (36,000 cases) |
| Current Standard | Chemotherapy; no approved ADCs for HER3‑positive NPC |
| Peak Sales Forecast (China) | ¥2.5‑3.5 billion (~US$340‑480 million) by 2032 |
| Market Share Target | 40‑50% of eligible HER3‑positive patients |
| Competition | First‑mover advantage; rivals (e.g., Daiichi’s HER3 ADC) still in early development |
- Regulatory Path: NDA acceptance triggers 6‑month Priority Review; potential approval Q3 2026
- BMS Partnership: Global Phase III program expansion funded by BMS; China approval will inform ex‑China regulatory strategy
- Next Catalysts: U.S. Phase III readout for other indications H2 2026; potential for global filing in HER2‑negative breast cancer
Forward‑Looking Statements
This brief contains forward‑looking statements regarding iza‑bren’s regulatory review timeline, commercial potential, and global development. Actual results may differ materially due to risks including NMPA approval outcomes, competitive dynamics, and BMS partnership execution.-Fineline Info & Tech