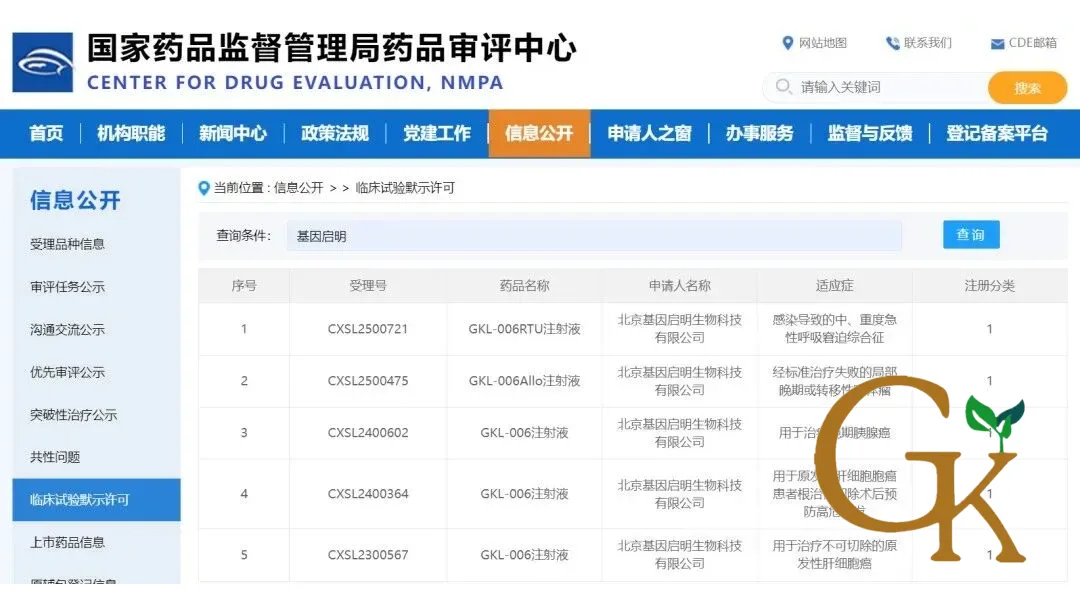
Beijing Gene Key Life Technology Co., Ltd. announced that its GKL‑006RTU Injection, China’s first novel allogeneic ready-to-use iNKT cell therapy, received IND approval from the National Medical Products Administration (NMPA) for moderate-to-severe Acute Respiratory Distress Syndrome (ARDS) caused by infection, marking the company’s fifth IND approval and its first non-oncology indication.
Regulatory Milestone
| Item | Detail |
|---|---|
| Product | GKL‑006RTU Injection (allogeneic iNKT cell therapy) |
| Company | Beijing Gene Key Life Technology Co., Ltd. |
| Agency | NMPA (China) |
| Application Type | IND approval |
| Indication | Moderate-to-severe ARDS caused by infection |
| Significance | First allogeneic ready-to-use iNKT cell injection in China; Company’s 5th IND; First non-oncology indication |
| Next Step | Phase I/II trial initiation Q1 2026 |
Technology Profile
- Mechanism: Invariant Natural Killer T (iNKT) cells possess dual anti-tumor activity and immune regulatory functions
- Platform: Allogeneic, ready-to-use formulation eliminates need for patient-specific manufacturing
- Innovation: First iNKT product targeting infectious disease vs. traditional oncology focus
- Pipeline: Gene Key has developed multiple iNKT cell therapeutics for various tumors; ARDS program leverages immunomodulatory capabilities
Market Context & Outlook
| Metric | Value |
|---|---|
| China ARDS Incidence | ~300,000 cases annually (infection-related) |
| Moderate-to-Severe Cases | ~120,000 patients (40% of total) |
| Current Standard | Corticosteroids, supportive care; no approved cell therapies |
| Mortality Rate | 30‑40% for moderate-to-severe ARDS |
| Cell Therapy Market | ¥15 billion (US$2.1 billion) in 2024 |
| Peak Sales Forecast | ¥1.5‑2.5 billion (US$200‑340 million) by 2032 |
| Competitive Edge: First-mover in iNKT for ARDS; allogeneic platform enables scale and affordability |
- Clinical Rationale: iNKT cells can modulate cytokine storms characteristic of ARDS while avoiding overt immunosuppression
- Regulatory Path: ARDS qualifies for breakthrough designation due to high unmet need; potential for accelerated review
- Manufacturing: Ready-to-use format reduces treatment delay from weeks to hours, critical in acute ARDS setting
Forward‑Looking Statements
This brief contains forward‑looking statements regarding GKL‑006RTU’s clinical development, market potential, and manufacturing scale-up. Actual results may differ materially due to risks including clinical trial outcomes, safety profiles, and competitive dynamics in cell therapy.-Fineline Info & Tech