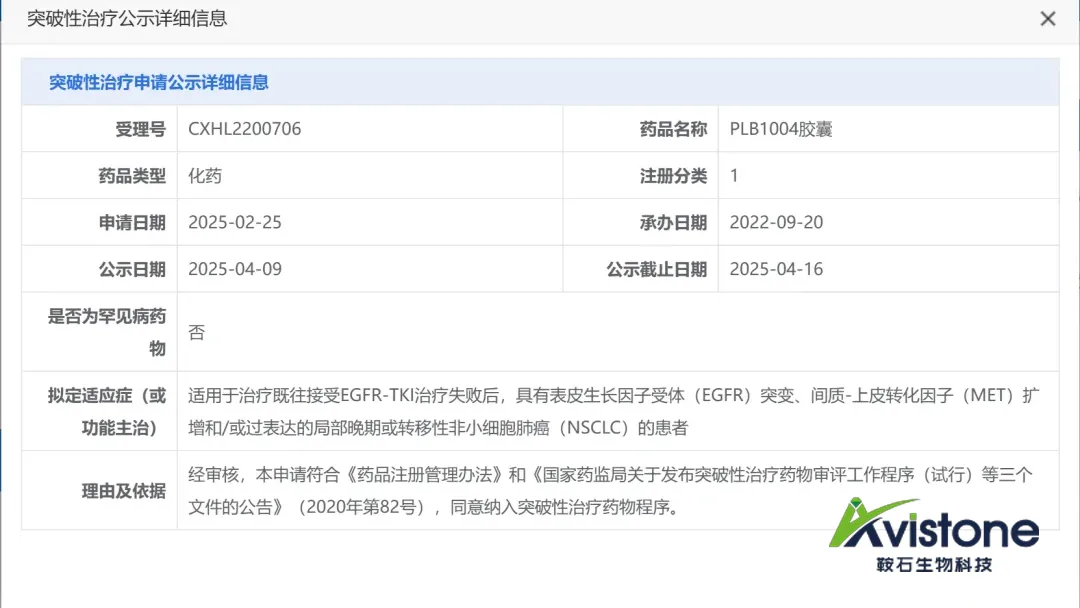
China’s Center for Drug Evaluation (CDE) has granted breakthrough therapy designation (BTD) to Beijing Avistone Pharmaceuticals Biotechnology Co., Ltd. for its c-MET inhibitor vebreltinib in combination with the EGFR inhibitor PLB1004. The designation is for treating locally advanced or metastatic non-small cell lung cancer (NSCLC) patients with EGFR mutations, MET amplification, and/or MET overexpression following failure of prior EGFR tyrosine kinase inhibitor (EGFR-TKI) therapy.
Vebreltinib and PLB1004: Mechanism and Development
Vebreltinib, a selective c-MET inhibitor, was originally developed by CrownBio and co-developed by Apollomics Inc. and Pearl Bio (acquired by Avistone in 2022). It received approval in China in November 2023 for MET exon 14 skipping NSCLC. PLB1004, an in-house-developed small-molecule EGFR inhibitor by Avistone, demonstrates strong blood-brain barrier penetration and potent activity against EGFR exon 20 insertion mutations (EGFR 20ins) and classic mutations such as Del19, L858R, and T790M.
Clinical Implications
The combination therapy’s BTD highlights its potential to address treatment resistance in NSCLC patients with complex genetic profiles. By combining vebreltinib’s MET-targeting capabilities with PLB1004’s robust EGFR inhibition and blood-brain barrier penetration, the therapy aims to improve outcomes for patients with limited treatment options.-Fineline Info & Tech