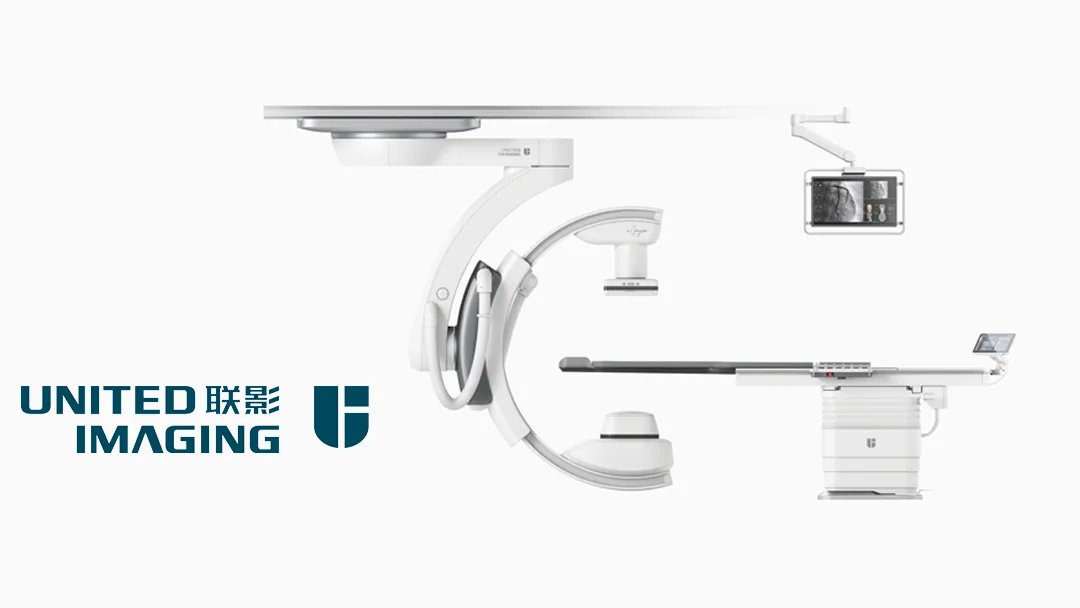
China-based United Imaging Healthcare (UIH, SHA: 688271) announced that it has received marketing approval from the US Food and Drug Administration (FDA) for its first interventional X-ray system, the uAngio AVIVA. This marks a significant milestone in the company’s global expansion and technological leadership.
System Features and Innovation
The uAngio AVIVA is an intuitively bionic ceiling-mounted interventional X-ray system. It integrates intelligent robotics, voice control, vision, and imaging technologies to actively complete tasks such as target part recognition, motion trajectory planning, exposure parameter calculation, and collision risk warning. This advanced system allows for full lab coverage and easy tableside access, simplifying navigation around the complex medical equipment typically found in interventional sites.-Fineline Info & Tech