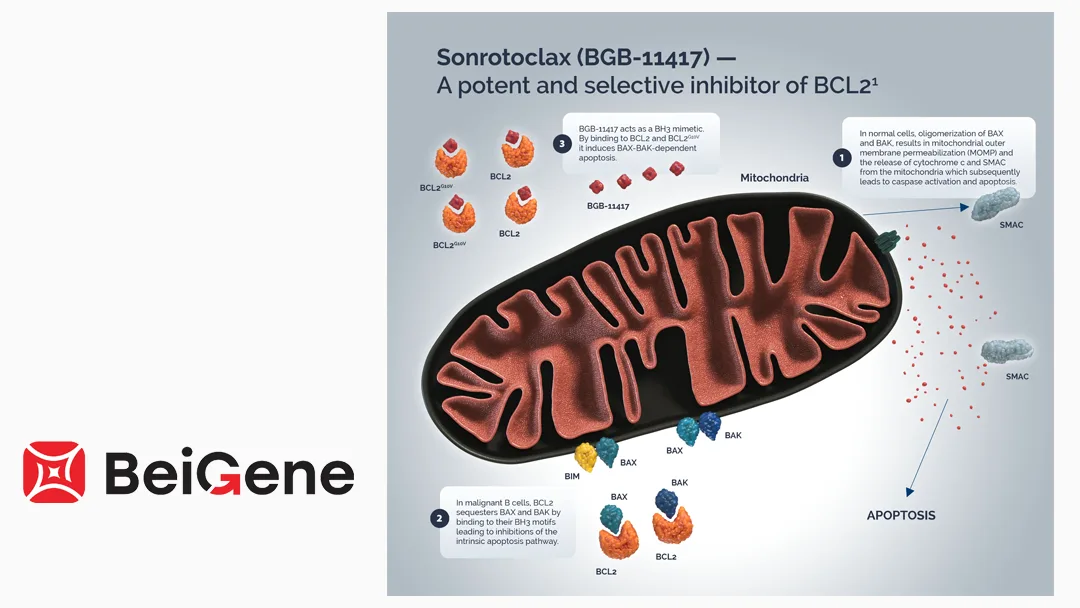
BeiGene, Ltd. (NASDAQ: ONC, HKG: 6160, SHA: 688235), a China-based oncology specialist proposing to change its name to BeOne Medicines Ltd., announced that the National Medical Products Administration (NMPA) has accepted another indication approval filing for its BCL2 inhibitor sonrotoclax. The drug is filed for the treatment of mantle cell lymphoma (MCL) in patients previously treated with anti-CD20 therapy and BTKi therapy. This indication has been awarded priority review status.
Prior NDA Acceptance
Last month, the NMPA also accepted a New Drug Application (NDA) for sonrotoclax as a treatment for previously treated chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), with priority review status granted.-Fineline Info & Tech