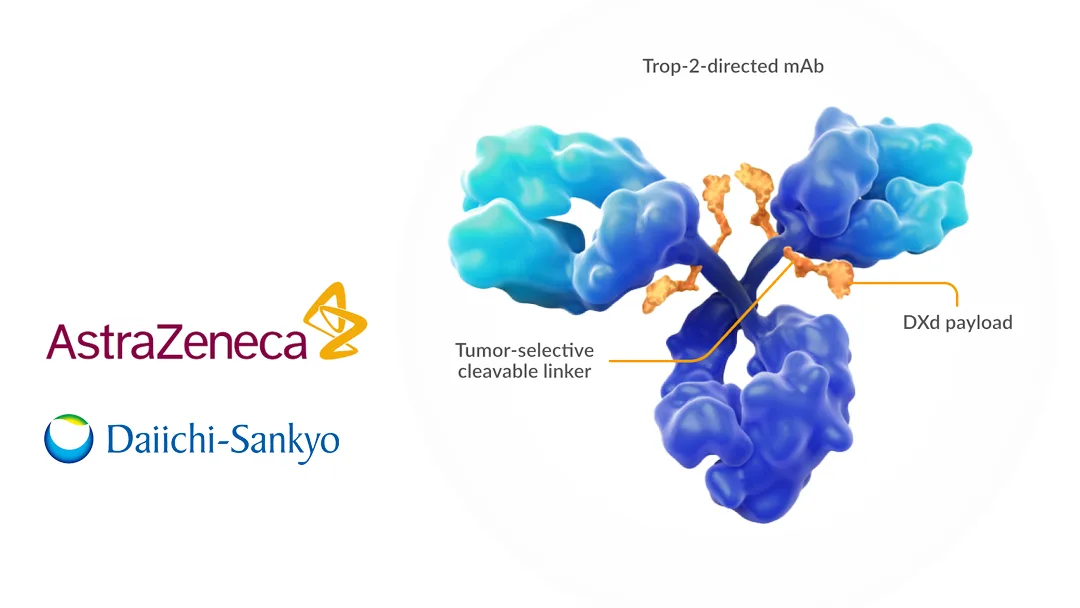
The US Food and Drug Administration (FDA) this week approved Datopotamab deruxtecan (Dato-DXd), a TROP-2-targeted antibody drug conjugate (ADC) co-developed by UK-based AstraZeneca (NASDAQ: AZN) and Japan’s Daiichi Sankyo (TYO: 4568). The approval is for the treatment of adults with locally advanced or metastatic EGFR-mutated non-small cell lung cancer (NSCLC) who have previously received EGFR-targeted therapy and platinum-based chemotherapy.
Clinical Trial Results
The FDA approval was granted under Priority Review and Breakthrough Therapy designation, based on subgroup results from the TROPION-Lung05 Phase II and TROPION-Lung01 Phase III trials. Across both studies (n=114), Datroway achieved a confirmed objective response rate (ORR) of 45% (by blinded independent central review/BICR), with 4.4% complete response (CR), 40% partial response (PR), and a median duration of response (DoR) of 6.5 months.
Previous Approvals
The drug previously received approvals for HR+/HER2- breast cancer in Japan (December 2024) and the US (January 2025), as well as in the European Union (April 2025).-Fineline Info & Tech