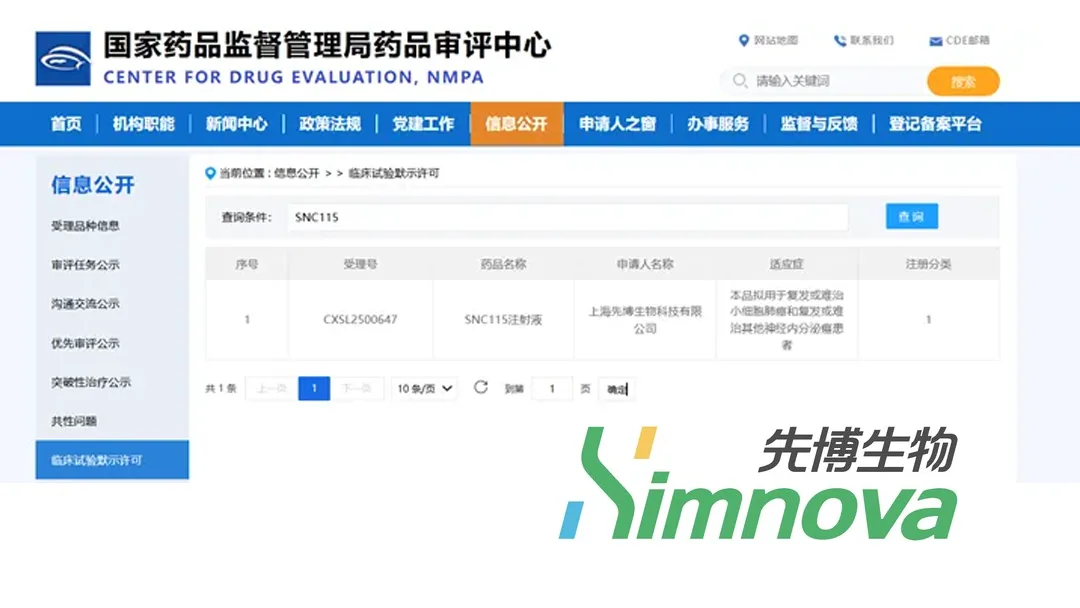
Shanghai Simnova Biotech Co., Ltd. announced that its self‑developed anti‑DLL3 chimeric antigen receptor T‑cell (CAR‑T) injection, SNC115, has received formal clinical‑trial approval from the National Medical Products Administration (NMPA). The IND clearance paves the way for a first‑in‑China study of a DLL3‑directed CAR‑T therapy in patients with relapsed or refractory small‑cell lung cancer (SCLC) and other neuroendocrine malignancies.
Key Highlights
| Item | Detail |
|---|---|
| Product | SNC115 – anti‑DLL3 CAR‑T cell injection |
| Regulatory Milestone | NMPA clinical‑trial approval (IND) – 30 Oct 2025 |
| Indication | Relapsed/refractory SCLC and selected neuroendocrine cancers |
| Target Rationale | DLL3 is highly expressed on tumor cells but minimally on normal tissue, offering a high therapeutic index |
| First‑in‑China | First DLL3‑targeted CAR‑T product cleared for clinical development in the Chinese market |
| Study Design | Multicenter Phase I/II, dose‑escalation followed by expansion; primary endpoints: safety, tolerability, objective response rate |
| Timeline | First patient in (FPI) expected Q1 2026; data read‑out anticipated late 2027 |
Why DLL3?
- Tumour‑Specific Expression – DLL3 is a Notch‑pathway ligand that is over‑expressed on > 80 % of SCLC cells while virtually absent in healthy adult tissues.
- Therapeutic Window – The stark differential expression makes DLL3 an ideal antigen for CAR‑T targeting, reducing off‑tumour toxicity.
- Unmet Need – Relapsed/refractory SCLC has a 5‑year survival < 5 %; current options are limited to chemotherapy and limited‑duration immunotherapy.
About Simnova
- Founded: 2019 as a Simcere Pharmaceutical subsidiary; spun off as an independent biotech in 2021.
- R&D Footprint: Dual research hubs in Shanghai (China) and Boston (USA).
- Pipeline Focus: Cell‑based immunotherapies for solid tumours, with a particular emphasis on novel tumor‑associated antigens (DLL3, B7‑H3, etc.).
Clinical Development Path
- Phase I Dose‑Escalation – Establish maximum tolerated dose (MTD) and assess cytokine release syndrome (CRS) profile.
- Phase II Expansion – Enroll up to 60 patients with SCLC or neuroendocrine tumors; evaluate objective response rate (ORR) and duration of response (DoR).
- Regulatory Strategy – Parallel submission for accelerated approval pathways (China’s “Priority Review” and potential U.S. IND filing in 2026).
Market Outlook
- China Oncology Landscape – CAR‑T therapies for hematologic malignancies have already captured > $1 B in sales; solid‑tumor CAR‑T is the next frontier.
- Revenue Potential – Analysts estimate a peak‑year market of $500 M–$800 M for a successful DLL3‑CAR‑T in SCLC, given the high incidence (> 30,000 new cases annually in China).
- Strategic Partnerships – Simnova may seek co‑development or licensing deals with global pharma to expand SNC115 beyond China.
Forward‑Looking Statements
This brief contains forward‑looking statements that involve risks and uncertainties, including regulatory approvals, clinical trial outcomes, and commercial prospects. Actual results may differ materially from those expressed herein.-Fineline Info & Tech