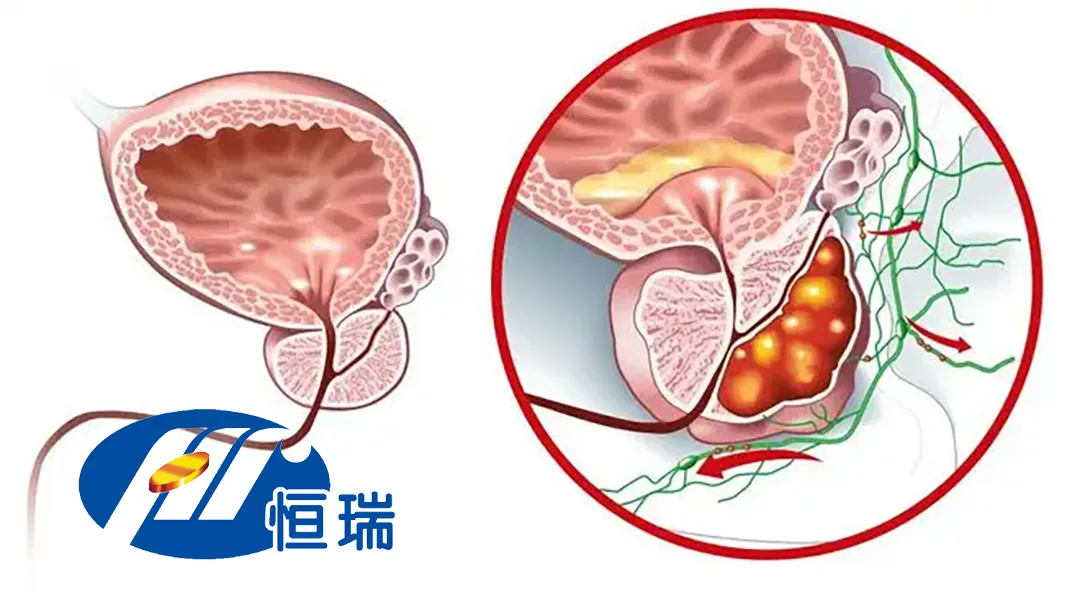
China‑based Jiangsu Hengrui Pharmaceuticals Co., Ltd. (SHA: 600276, HKG: 1276) announced today that the China National Medical Products Administration (NMPA) has approved a clinical study evaluating rezvilutamide in combination with HS‑20093 for patients with advanced prostate cancer. The move positions Hengrui at the forefront of next‑generation androgen‑receptor (AR) blockade paired with antibody‑drug conjugate (ADC) technology.
Why This Combination Is Clinically Promising
| Element | Feature | Clinical Significance |
|---|---|---|
| Rezvilutamide | Second‑generation AR inhibitor, potent AR blockade, no agonistic activity | Superior efficacy over first‑gen AR agents for metastatic hormone‑sensitive prostate cancer (mHSPC) |
| HS‑20093 | B7‑H3‑targeted ADC, previously studied in lung, sarcoma, and head‑and‑neck cancers | Expands therapeutic reach to solid tumors, potentially synergizing with AR inhibition |
| Combination Synergy | Dual engagement of tumor growth pathways | May overcome resistance mechanisms seen with monotherapy |
Key Milestones & Next Steps
- NMPA approval (Nov 2025) – Enables Phase I/II trial start in mainland China.
- Trial design – Multi‑center, dose‑escalation followed by safety‑and‑efficacy evaluations.
- Patient enrollment – Target population: high‑volume metastatic prostate cancer patients, with optional extension to CRPC (castration‑resistant).
- Global expansion – Upon favorable safety signals, the study will seek regulatory approval pathways in the U.S. and other markets.
Regulatory & Market Implications
- Strategic advantage for Hengrui – First‑in‑class AR/ADC combination trial in Asia, unlocking a rapid entry to a high‑revenue oncology segment.
- Share performance – Hengrui’s Shanghai and Hong Kong shares responded positively, reflecting investor confidence in the combination platform.
- Pipeline diversification – Reinforces Hengrui’s portfolio of approved agents (rezvilutamide for 2022‑approved mHSPC) and ADC candidates (HS‑20093) across multiple solid‑tumor indications.-Fineline Info & Tech