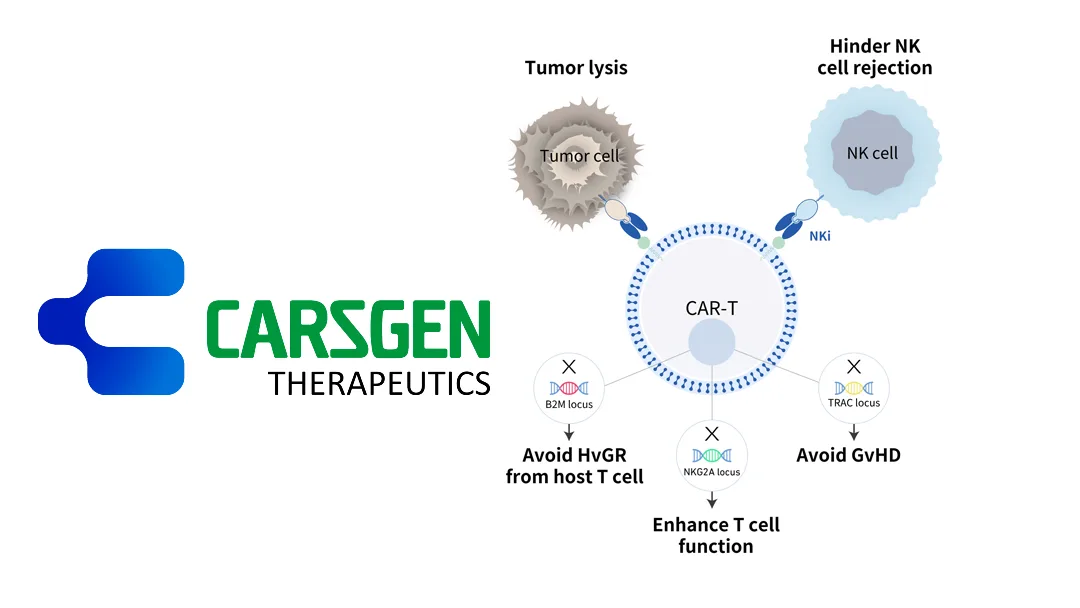
China‑based CARsgen Therapeutics Holdings Limited (HKG: 2171) unveiled clinical data for two allogeneic CAR‑T candidates built on its proprietary THANK‑u Plus platform:
- CT0596 – a BCMA‑targeting product in relapsed/refractory multiple myeloma (R/R MM)
- CT1190B – a dual‑targeting CD19/CD20 product in relapsed/refractory non‑Hodgkin lymphoma (R/R NHL)
Both programs demonstrated favorable safety profiles and encouraging efficacy signals in early‑stage studies.
Key Highlights
• CT0596: 8 patients – 83 % overall response rate (ORR), 3 of 8 achieving CR/sCR, 6 MRD‑negative by week 4.
• CT1190B: 8 patients – 83.3 % ORR (4 CR, 1 PR), no ICANS or GVHD observed.
• Safety: No dose‑limiting toxicities, treatment discontinuations or deaths reported.
CT0596: Allogeneic BCMA‑CAR‑T in Relapsed/Refractory Multiple Myeloma
- Objective Response Rate (ORR): 5/8 patients (62.5 %) achieved partial response or better, including 3 CR/sCR post‑full lymphodepletion.
- Minimal Residual Disease: 6/8 patients attained MRD‑negativity at week 4.
- Expansion & Persistence: All 8 patients showed CAR‑T expansion; no dose‑limiting toxicities (DLT), CRS, or fatalities.
- Safety: No CRS, grade ≥ 3 cytokine release syndrome, or other adverse events reported.
CT1190B: Dual‑Target CD19/CD20 CAR‑T in Relapsed/Refractory NHL
| Lymphoma Subtype | Lymphodepleting Regimen | Response | Notes |
|---|---|---|---|
| Follicular (3 pts) | F30 × 3 days + C500 × 3 days | CR (3/3) | All achieved CR. |
| Mantle Cell (2 pts) | F30 × 3 days + C1000 × 2 days (recommended) | CR (2/2) | |
| Diffuse Large B‑cell (6 pts) | Same | 4 CR, 1 PR (83.3 % ORR) |
Safety Profile
- Cytokine Release Syndrome (CRS), cytopenia, and infections were the primary adverse events.
- No cases of ICANS or GVHD were observed, underscoring the platform’s safety advantage.
Why These Findings Matter
CARsgen’s allogeneic platform addresses two critical hurdles for CAR‑T therapy—manufacturing scalability and cost containment—while preserving therapeutic potency. The early data suggest that CT0596 and CT1190B could expand the CAR‑T market into the R/R MM and NHL segments that currently rely heavily on autologous products.-Fineline Info & Tech